Building a coarse-grained model of the bacterial nucleoid
Research project of Maeva Fourny, student of the Soft Nanoscience program of GS@UGA, at the Liphy, Grenoble.
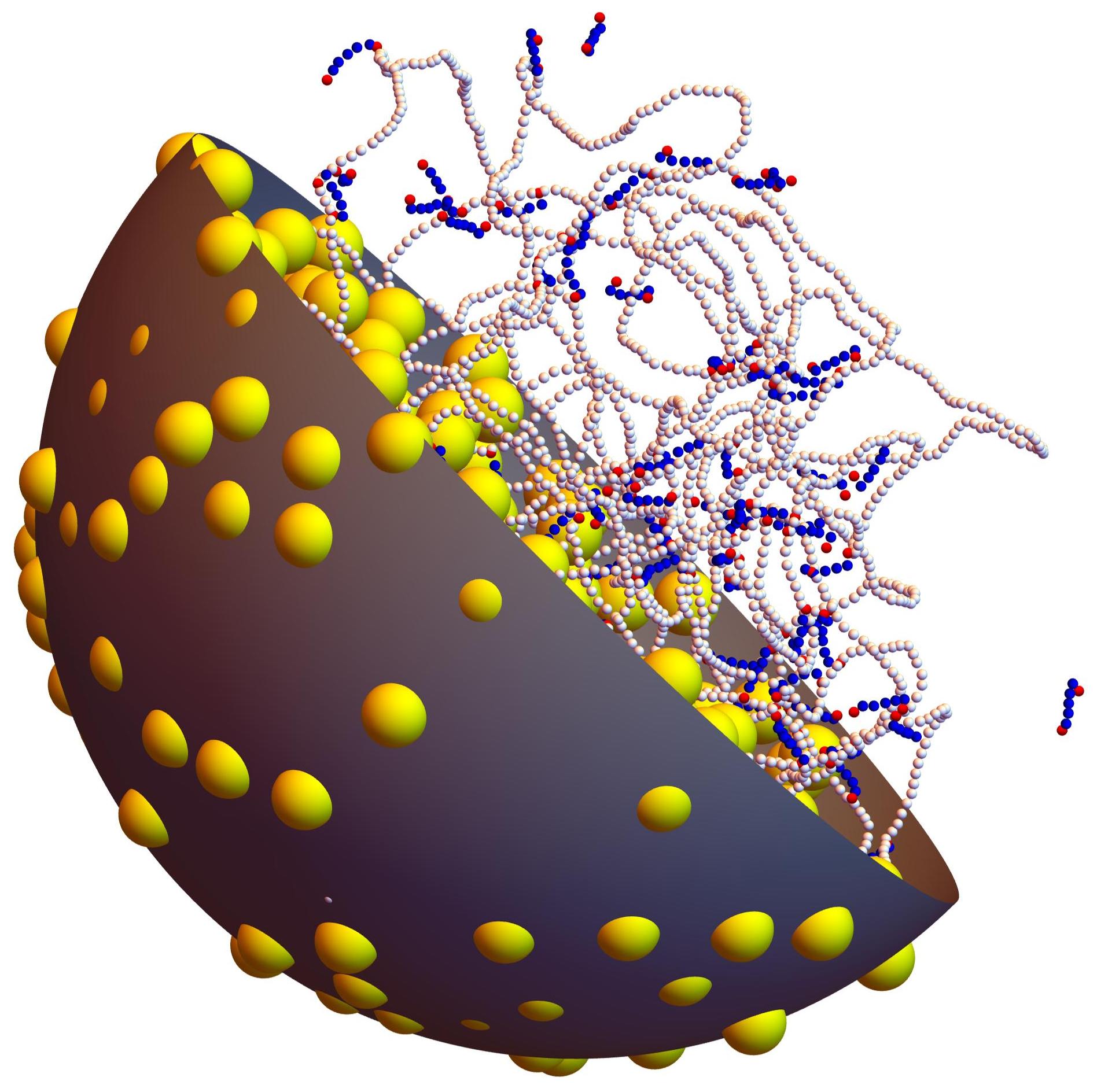
The dynamics of the chromosomal DNA of bacteria is arguably simpler than that of eukaryotic cells. Indeed, it is not separated from the rest of the cytoplasm by the nuclear envelope, and its compaction requires fewer levels than the complex hierarchy of mechanisms observed for eukaryotic DNA. Nonetheless, the organization of the bacterial nucleoid (the region of the cell that contains the genomic DNA, as well as several thousands of associated proteins) is still poorly understood and fundamental questions lack an answer, despite the long-lasting efforts of many groups. For example, the nature of the mechanism leading to the 1000-fold compaction of the DNA inside the nucleoid and its separation from the rest of the cytoplasm is still poorly understood. Moreover, the DNA of most bacteria is circular and negatively supercoiled, but the role of supercoiling and its interferences with fundamental biological processes, like DNA compaction, replication or transcription, are the matter of ongoing debates.
The purpose of the proposed internship is precisely to better understand the dynamics of the bacterial nucleoid through coarse-grained molecular modeling and simulations. We recently demonstrated that such models suggest that the principal mechanism for the formation of the bacterial nucleoid is a segregative phase separation driven by the demixing of the DNA and other non-interacting globular macromolecules present in the cytoplasm, presumably ribosomes. The proposed work aims at elaborating further on these results and refining the current models, in order to gain deeper insight into the formation of the nucleoid, the role of supercoiling, and the unavoidable interactions between all mechanisms that take place in the cell. It will therefore consist in building a coarse-grained model of the cytoplasm with all mandatory ingredients, namely supercoiled DNA, non-interacting crowding macromolecules, DNA-bound proteins and molecular motors, and launching simulations over the long time scales needed to investigate these mechanisms.
Published on December 20, 2023
Updated on September 4, 2024