Soft tissue connects, surrounds or supports internal organs and bones. They exhibit a large variety of microstructures and mechanical properties, which vary from organ to organ and according to patho-physiological conditions.
In these tissues, cells are dispersed away from one another and in a collagen-based matrix, the so-called extra-cellular matrix (ECM). Long-range coordination of cell behaviors has been observed in these tissues. They can be explained by the secretion of a chemical signal by a cell, its transport by diffusion (and possibly
other undocumented contributions) and detection by the distant cell. An alternative means of communication is the transmission of mechanical signals through the network.
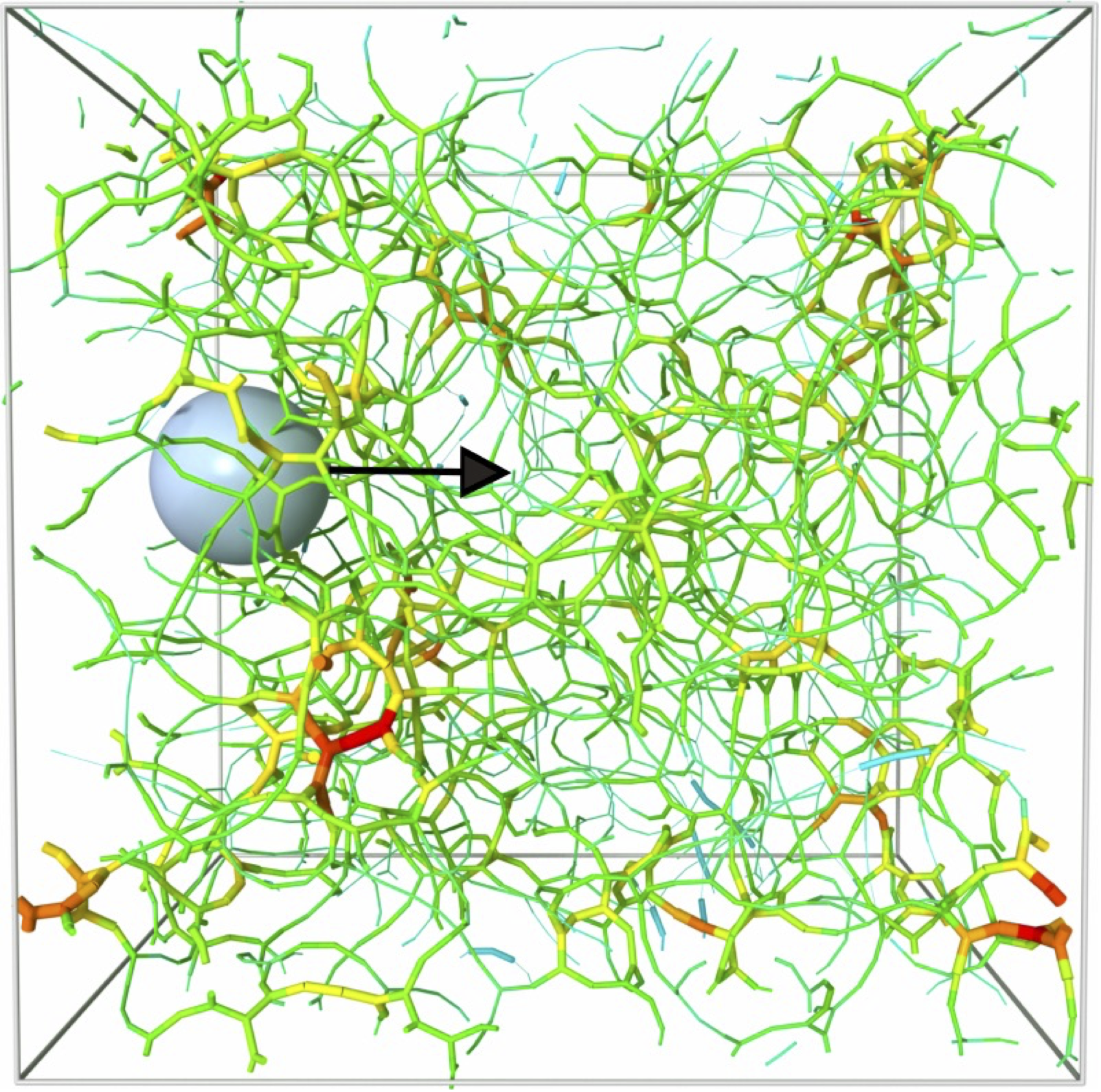
There is a recent recognition that many biochemical signals emitted by cells are not released in the extracellular fluid directly but are often present within extracellular vesicles (EVs) and many challenges remain to understand this form of intercellular communication. A major bottleneck is to understand how these vesicles can be transported within an extracellular network whose apparent average pore size is significantly smaller than the size of the EVs themselves. There is therefore an important need for a microscale understanding of the mechanics of this transport through an ECM having a given architecture.
In this internship we will use coarse-grained molecular dynamics simulation of a model collagen gel to determine the key physical mechanisms controlling the matrix remodeling and memory effects when a large particle is transported through the matrix. The embedded nanoparticle will be larger than the network pore-size and will be subjected to a constant force. We will characterize the subsequent recovery of the network microstructure as a function of temperature and force and determine i) the degree of plasticity of the network and ii) the characteristic rearrangement time scales.
The internship candidate will strongly interact with our collaborators at LiPhy (J. Etienne and G. Cappello)
References
[1] E. Wershof, et al. “Matrix feedback enables diverse higher-order patterning of the extracellular
matrix”. PLOS Computational Biology 15.10 (Oct. 2019).
[2] I. Levental, et al. “Soft biological materials and their impact on cell function”. en. In: Soft Matter 3.3 (2007), pp. 299–306.
[3] M. Yáñez-Mó, et al. “Biological properties of extracellular vesicles and their physiological functions”. Journal of Extracellular Vesicles 4 (1 2015), p. 27066.
[4] G. van Niel, et al. “Challenges and directions in studying cell–cell communication by extracellular vesicles”. Nat Rev Mol Cell Biol 23 (5 2022), pp. 369–382.
[5] S. Lenzini et al. “Matrix mechanics and water permeation regulate extracellular vesicle transport”. In: Nat.Nanotechnol. 15 (3 2020), pp. 217–223.
[6] A. Allard, M. Bouzid et al. “Actin modulates shape and mechanics of tubular membranes”. In: Science advances 6.17 (2020), eaaz3050.
[7] M. Bouzid et al. “Elastically driven intermittent microscopic dynamics in soft solids”. In: Nature communications
8.1 (2017), pp. 1–8.
Webpage
https://bouzidmehdi.wordpress.com/
Program and expected skills
Program: M1Soft Nanosciences or M1 Applied Mechanics
Expected skills:
Backgrounds in statistical physics, soft matter or mechanical engineering are expected. Interested in performing numerical simulations. Knowledge in numerical modeling (Python, C, C++,...) recommended.