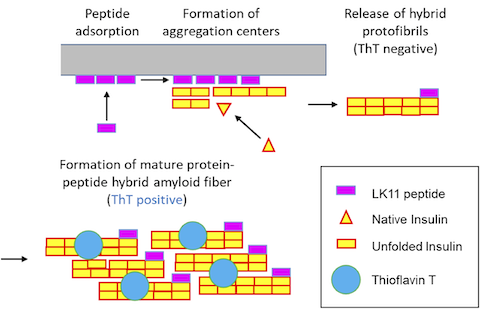
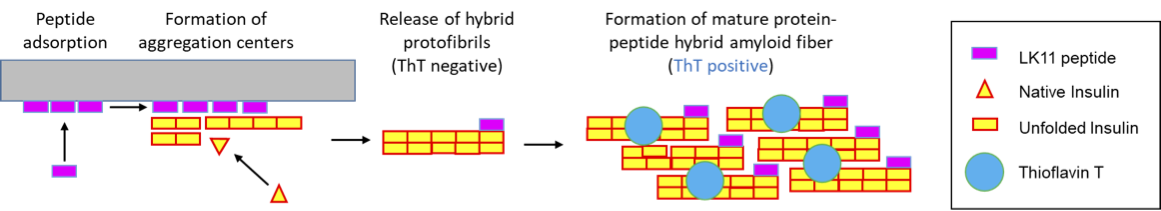
Human insulin is a therapeutic protein widely used to control blood glucose levels in diabetic patients. But insulin aggregation in plastic tubings reduces its bio-activity. The aggregates formed are called amyloid fibers.
Context :
Human insulin is a therapeutic protein widely used to control blood glucose levels in patients who have type 1 or 2 diabetes, but insulin aggregation in plastic tubings reduces its bio-activity and clogs medical devices. The aggregates formed are of a specific type, called amyloid fibers, which are characterized by a core of stacked beta-sheets that can retain a fluorescent dye, Thioflavin T (ThT).
Recent studies demonstrated that insulin aggregation on hydrophobic surfaces can be accelerated by peptides of sequence (LK)nL that form beta-sheets on hydrophobic surfaces. These peptides have a dual effect on insulin aggregation kinetics (Chouchane et al. 2015). At low concentration, surface-associated peptides accelerate insulin aggregation whereas at high concentrations, peptides in solution delay the formation of insulin amyloid fibers.
This work aims at studying the interaction between insulin and peptides on plastic surfaces and the mechanisms of fibrillation, using labeled proteins, FITC-insulin and TAMRA-(LK)5L to monitor insulin and peptides at different locations and quantify them.
Proposed subject and expected results :
We recently showed (i) that when insulin comes into contact with a (LK)nL coated surface, aggregates form and are released in solution very rapidly and (ii) that a substantial fraction of the peptides leave the surface and become part of hybrid amyloid fibers. Formation of hybrid protein-peptide aggregates was evidenced by FRET and self-quenching fluorescence phenomena.
The aims of the project are :
• To further characterize the size and abundance of the aggregates that are released rapidly from the surface
• To understand how fast they acquire the ability to bind Thioflavin T
• To study whether (LK)nL in solution interfere with the formation of Thioflavin T-positive aggregates
• To use FRET and self-quenching data to build a model of the initial aggregates
Trainee's work :
• Conduct a bibliography study on the mechanisms of amyloid formation and the importance of protofibrils, especially in the case of insulin
• Perform experiments at the bench using fluorescent multiwell plate assays and other fluorescent-based techniques
• Participate in the modeling of fluorescent signal kinetics, incorporating informations from FRET and self-quenching
• Participate in the design of future experimental setups to characterize protein aggregates released from the surface.
References :
K. Chouchane, C. Vendrely, M. Amari, K. Moreaux, F. Bruckert, M. Weidenhaupt (2015). Dual effect of (LK)nL peptides on the onset of insulin amyloid fiber formation at hydrophobic surfaces. J. Phys. Chem. B, 119 : 10543−10553
Xiaoli Qu Master 2 (NSB) internship (2022). Measuring the exchange rates of insulin and inhibitory peptides on plastic surface and formation of hybrid amyloid fibers